Principles of plastid reductive evolution illuminated by non-photosynthetic chrysophytes
Richard G Dorrell, Tomonori Azuma, Mami Nomura, Guillemette Audren de Kerdrel, Lucas Paoli, Shanshan Yang, Chris Bowler, Ken-ichiro Ishii, Hideaki Miyashita, Gillian Gile, Ryoma Kamikawa
Abstract
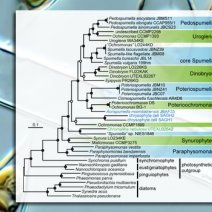
The division of life into producers and consumers is blurred by evolution. For example, eukaryotic phototrophs can lose the capacity to photosynthesise, although they may retain vestigial plastids that perform other essential cellular functions. Chrysophyte algae have undergone a particularly large number of photosynthesis losses. Here, we present the first plastid genome sequence from a non-photosynthetic chrysophyte, “Spumella” sp. NIES-1846, and show that it has retained a nearly identical set of plastid-encoded functions as apicomplexan parasites.
Our transcriptomic analysis of twelve different photosynthetic and nonphotosynthetic chrysophyte lineages reveals remarkable convergence in the functions of these non-photosynthetic plastids, along with informative lineage-specific retentions and losses. At one extreme, Cornospumella fuschlensis retains many photosynthesisassociated proteins, although it appears to have lost the reductive pentose phosphate pathway and most plastid amino acid metabolism pathways. At the other extreme, Paraphysomonas lacks plastid-targeted proteins associated with gene expression and all metabolic pathways that require plastid-encoded partners, indicating a complete loss of plastid DNA in this genus. Intriguingly, some of the nucleus encoded proteins that once functioned in the expression of the Paraphysomonas plastid genome have been retained. These proteins were likely to have been dualtargeted to the plastid and mitochondria of the chrysophyte ancestor, and are uniquely targeted to the mitochondria in Paraphysomonas.
Our comparative analyses provide new insights into the process of functional reduction in non photosynthetic plastids.
PNAS published ahead of print March 14, 2019. doi.org/10.1073/pnas.1819976116